Pharma 4.0 is all about bringing a new era of digital transformation and automation, powered by innovative, advanced technologies, into the life sciences industry. The key is to build on various levels of digital maturity, which are governed by a holistic strategy that follows well-established ICH quality guidelines. Pharma 4.0 is driven by the operating model presented in the figure below. This is no longer a choice but a necessity as automation is inevitable.
There are 4 basic elements of the Pharma 4.0 model: resources, information systems, culture, organization, and processes – all functioning together seamlessly with no boundaries. This enterprise-wide end-to-end vision is key for any organization to leap forward on the status of the digital maturity spectrum. Essential to all of this, of course, is to have an effective knowledge management mechanism while establishing an optimal level of management oversight when and where it’s needed.
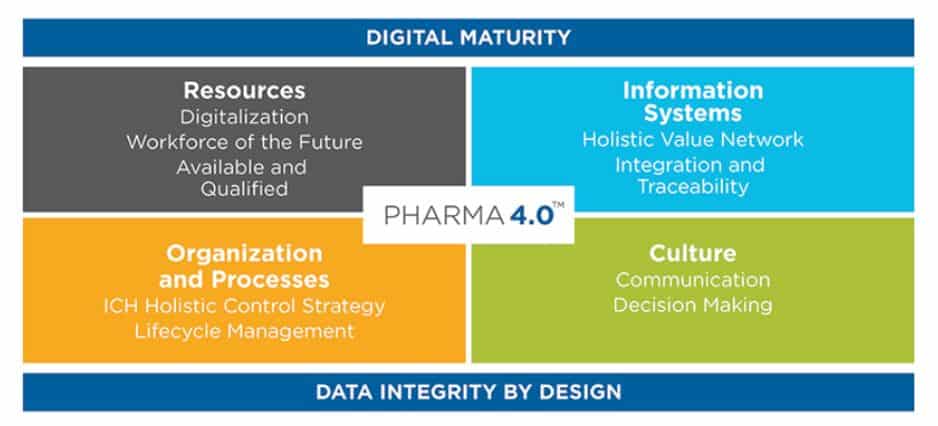
Figure 1: The “how to” steps of holistic control strategy are embedded in the Pharma 4.0™ operating model
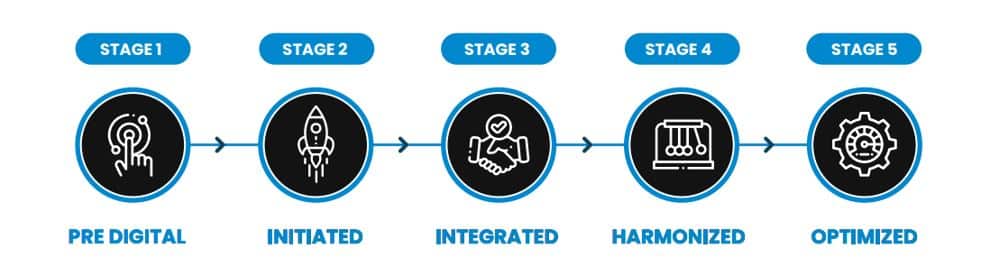
Let’s take a few minutes to look at the various stages of the digital maturity model. A good question to consider…Where is your organization on the model? There are 5 stages in the digital maturity model depicted below. Stage 1 is the pre-digital stage, where digital elements and automated elements coexist with the manual elements but are sporadic and are not really of good quality. Stage 2 is the initiated stage where the digital elements are growing, maturing and becoming actively managed. But all of this is happening in silos. And we all know that working in silos is not going to cut it if you’re going to talk about digitization and transformation. Stage 3 takes care of the silos because the silos are eliminated. We now have integration between the automated and manual processes, while automated and digital processes are being discussed by executives when it comes to making strategic decisions to meet the objectives of the organization. Stage 4, the harmonization stage, is when an organization actively asks questions like “Can we automate this process?” and “Why do we need to go to a manual process?” So more and more this has become part of the organization’s psyche and way of operating. Stage 5, what we all aim for in the life sciences world, is the optimized approach where everything is transformational. High priority digital elements that drive strategic value are part of the future, ethos, and critical success factors of the organization.
Given what we now know about the digital maturity model and the Pharma 4.0 journey, tune in to this webcast and see how AmplifyBio started on its journey to Pharma 4.0 and Digital Transformation.
References: