Verista to Showcase COUNTQ® Tray Inspection System at INTERPHEX 2025
PRESS RELEASE
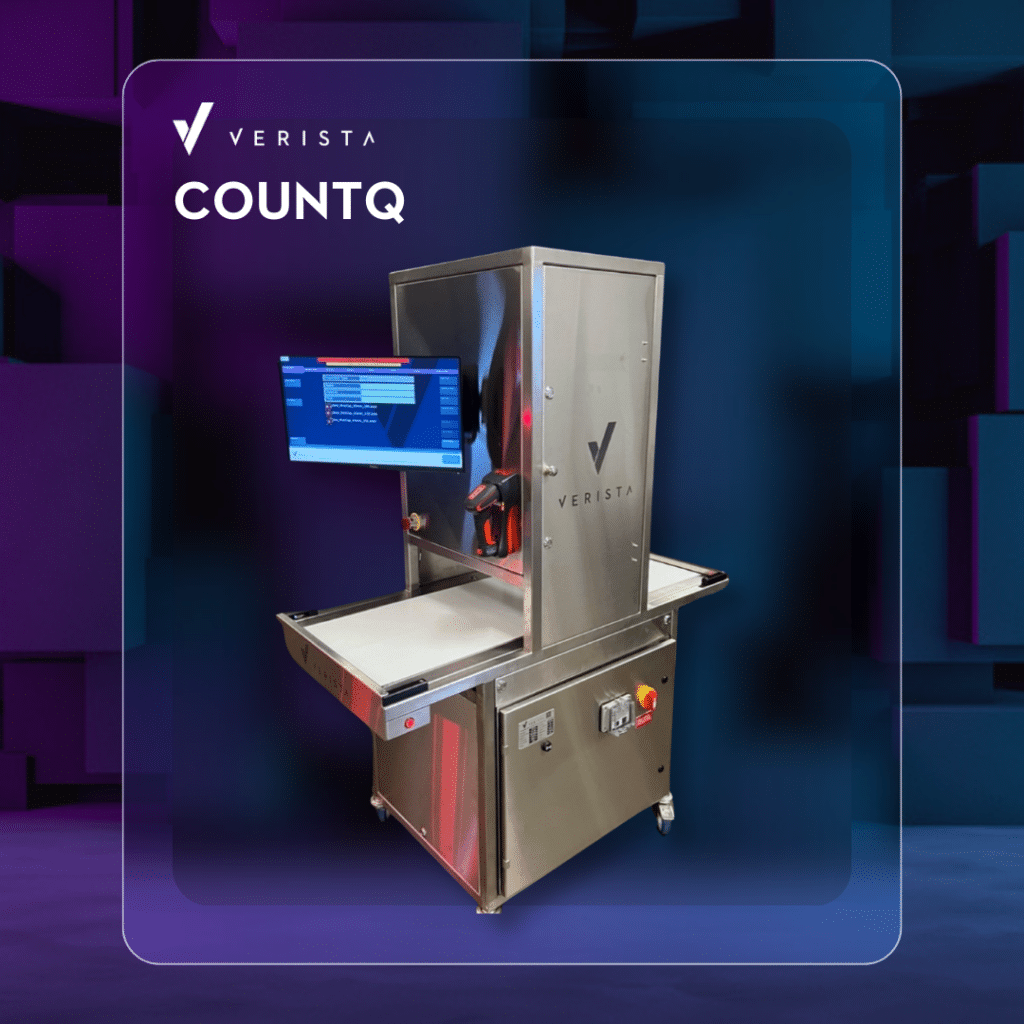
Verista, a leading provider of automation, compliance, and quality solutions for the pharmaceutical industry, is excited to announce its participation at INTERPHEX 2025, occurring April 1-3 at the Jacob Javits Center in New York City, NY, at booth 2860. Verista will be exhibiting its latest products and services, highlighting the COUNTQ® Tray Inspection System, an advanced AI-enabled inspection and reconciliation system designed to revolutionize the counting and verification of vials, syringes, ampoules, and other tray-loaded parts in pharmaceutical manufacturing. Verista’s technical experts will also be at the booth.
The COUNTQ® system delivers 100% electronic verification of product counts in just 15 seconds, significantly enhancing accuracy and throughput compared to manual methods. The system leverages deep learning AI technology for precise visual inspection, ensuring highresolution imaging with LED lighting. It offers a processing capacity of up to 100 million vials per year, supporting a standard fill line capacity of 300 vials per minute. The system is compatible with various tray types and product sizes, eliminating the need for tray templates and allowing for quick changeovers with unlimited recipe storage.
Verista’s COUNTQ® system is fully compliant with 21 CFR Part 11 regulations, providing a comprehensive audit trail. It has already made a significant impact in the industry, with multiple leading injectable manufacturers, including top pharmaceutical companies and major CMOs, implementing multiple units across their facilities. These installations have resulted in improved accuracy, higher throughput, reduced labor costs, and enhanced regulatory compliance.
“We are excited to demonstrate the capabilities of our COUNTQ® system at INTERPHEX 2025,” said Jim Evans, Director of the Vision & System Integration group at Verista. “We have been a leader in count reconciliation systems for over 20 years and this current product family represents a significant leap forward in inspection efficiency and accuracy, addressing critical challenges in pharmaceutical manufacturing.”
Verista’s technical experts will be available at booth 2860 to provide in-depth demonstrations of the COUNTQ® system and discuss its implementation in pharmaceutical manufacturing environments. Attendees will have the opportunity to learn how this technology can streamline operations, reduce labor costs, and ensure the highest quality standards in their facilities. For more information, please visit www.verista.com.
About Verista
Verista is a leading provider of automation, compliance, and quality solutions for the pharmaceutical, biotechnology, and life sciences industries. Headquartered in Fishers, Indiana, with additional offices in Pennsylvania, Massachusetts, and California, Verista specializes in services such as computer systems validation, commissioning and qualification (CQV), manufacturing and packaging automation, machine vision, and regulatory compliance. Leveraging innovative technologies and over 500 industry experts, Verista helps clients ensure data integrity, meet stringent regulatory requirements, optimize operations, and accelerate time-to-market for life-saving products. With a focus on digital transformation and operational excellence across the product lifecycle, Verista empowers organizations to deliver safe, high-quality medical products efficiently. For more information, visit www.verista.com.
Media Contact:
Kristin Ding
Marketing Manager
[email protected]